Nanoimager – Super Resolution Single Molecule Imaging
The Nanoimager is a revolutionary new single molecule microscope that redefines what is possible in super resolution single molecule imaging. It will allow you to capture the most sensitive single molecule fluorescence data using a simple to operate, compact benchtop instrument that will easily find a home in your lab.
The Nanoimager offers super resolution imaging, revealing cellular detail better then 20nm, breaking the diffraction limit of diffracted light. This provides levels of understanding not previously possible pertaining to the interaction and ultrastructure of molecules.
The Nanoimager is a high-throughput, purpose-built instrument with phenomenal specifications. It is the worlds first tailored wide field single molecule FRET solution that also offers super resolution imaging with a resolution better than 20nm.
Imaging Technology
To achieve super resolution imaging, the Nanoimager uses single molecule localisation. This is similar to other techniques such as Direct Stochastic Optical Reconstruction Microscopy (dSTORM) and Photoactivated Localisation Microscopy (PALM). These techniques involve localising subsets of fluorophores in consecutive frames 9<20m laterally and 50nm axially depending on the actual experiment) and then reconstructing an image fro the positions of the localisations.
Key Features
- Resolution, better than 20nm
- Single molecule tracking
- Temperature control including the ability to heat the microscope (>37°C).
- Large field of view (10x larger than the competition)
- Homogenous illumination
- Autofocus- Z lock capability
Design of the Nanoimager
The Nanoimager has been purpose-designed from the ground up and optimised for high throughput single molecule imaging. The elegant design overcomes the limitations of more traditional systems.
Design benefits of the Nanoimager include:
- Small footprint enables the system to be easily integrated in imaging facilities and high biosafety labs where space is a premium
- Internal vibration damping allows the system to be used on a typical desk or benchtop regardless of imaging mode
- Streamlined optical path minimises image aberrations
- Drift is minimised through the solid, compact from factor
Furthermore, the system features a closed design eliminating the chance of misalignment. Once your sample is prepared, you are ready to image allowing you to spend more time imaging and less time setting up. The closed design also precludes dirt and other contaminants, thus reducing maintenance.
Compact Size
With a footprint smaller than an A4 piece of paper, finding space to put the Nanoimager microscope in your laboratory will not be an issue.
The associated light engine which houses the lasers can be located on the bench or elsewhere like a PC tower. It is connected to the microscope via an umbilical cord and communicates via optical fibres.
The bespoke design with in-built vibration damping means there is no need for optical benches, laser labs or dedicated temperature-controlled rooms.
Imaging Modes
The Nanoimager offers a host of imaging modalities including:
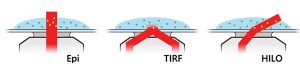
- Epifluorescence
- Total Internal Reflection Reflectance (TIRF)
- Highly Inclined and Laminated Optical Sheet (HILO)
- Single molecule FRET
- Super resolution imaging
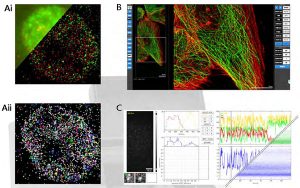
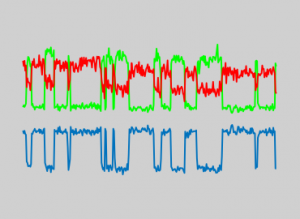
Data analysis and presentation. A. (i) conventional analysis and (ii) super resolution analysis. B. Rel time rendering of the super resolution images, with localisations able to be immediately filtered and quantitatively analysed. C. Real time analysis of smFRET traces (upper left) and population average (lower right).
Imaging Modes
The Nanoimager employs a latest generation sCMOS camera in conjunction with a high numerical aperture, high magnification oil immersion lens. This allows you to image at high temporal resolution, capturing full frame images in milliseconds. You can achieve even high temporal resolution by imaging a smaller area at up to 5kHz.
The hardware works synchronously with sophisticated software. The state-of-the-art scMOS camera, generates images that are superior, with less noise than alternative detectors such as EMCCD for most common applications such as super resolution.
Epifluorescence, TIRF and HILO imaging modes are all available with the Nanoimager. Each of these modes uses a different optical path. However, you can change between modes at the click of a button.
Epifluorescence
Epifluorescence or wide-field imaging is the most commonly used mode of fluorescence imaging. It shines a parallel beam of light up through the sample.
The Nanoimager combine high magnification (1 pixel = 117nm) and wide field of view in epifluorescence mode which other systems are unable to achieve. This is the preferred imaging mode for samples deeper than 10µm but can result in higher background noise from excited molecules outside the focal plane.
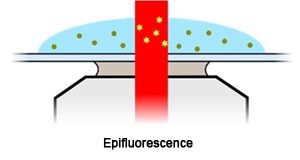
In epifluorescence mode the laser passes up through the sample exciting molecules deeper inside. It produces higher background from more out of focus molecules.
Total Internal Reflection Fluorescence (TIRF)
TIRF imaging with the Nanoimager produces images with the greatest signal to noise ratio. This mode only excites a 200nm deep section near the coverslip. Almost all excited molecules are in focus, while background signals are significantly reduced.
TIRF imaging is ideally suited to molecules attached to the surface or on a membrane.
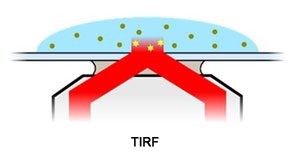
In TIRF mode the laser is incident above the critical angle for the refractive index change between cover slip and sample resulting in internal reflection. This excites only the thin evanescent region (approx 200nm) near the interface producing high signal to noise data.
Highly Inclined and Laminated Optical Sheet (HILO) Imaging
In this mode, the sample is imaged using a laser that impinges the sample at a large angle. This allows you to analyse to a depth of about 10µm with a signal-to-noise ratio only marginally below TIRF.
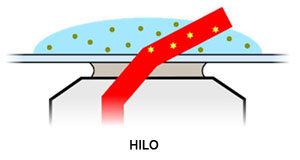
In HILO mode the laser is incident slightly below the critical angle so teh laser passes through the sample at a highly inclined angle. It is a compromise between epifluorescence and TIRF modes.
Simultaneous Dual Colour Imaging, Sequential Four Colour Imaging
The Nanoimager provides imaging in dual colour mode, supporting co-localisation studies and the capture of dynamic information from two different molecular species provided they are labelled with two spectrally different fluorphores. Dual colour imaging is also essential for smFRET experiments.
It is also able to image up to four different excitation colours sequentially or using a pattern of interlaced lasers.
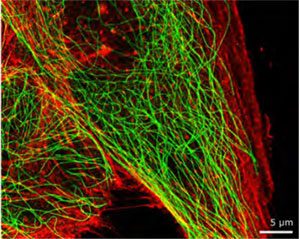
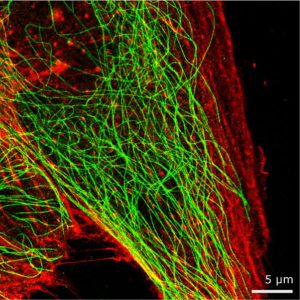
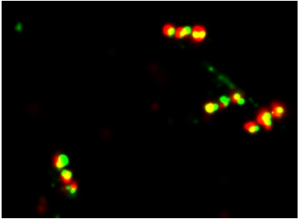
Dual colour super resolution image of an MDBK cell with microtubules (green) and actin (red).
Single Molecule Forster Resonance Energy Transfer (smFRET)
The Nanoimager is the worlds first commercially available solution for wide-field smFRET studies. This imaging mode allows you to measure in real time intramolecular distances in a single protein or nucleic acid or specific interactions between subunits in a protein complex.
smFRET imaging has wide reaching potential in areas such as:
- The effect of drugs on the conformational dynamics of enzyme binding sites
- The study of protein aggregation in neurodegenerative diseases
To infer binding constants, reaction pathways and dwell time distributions at the stochastic single molecule level, not obscured by ensemble averaging
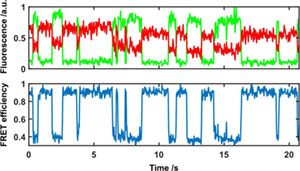
Real time conformational changes detected using smFRET.
Super Resolution Imaging
Super resolution imaging allows you to view cellular features down to 20nm and better depending on the sample thanks to single-molecule localisation technology. Imaging at this level provides unprecedented information about cellular interaction and ultrastucture.
Furthermore, the Nanoimager software allows complimentary analyses such as co-localisation and clustering which give you an in-depth quantitative analysis of interacting species and molecular function.
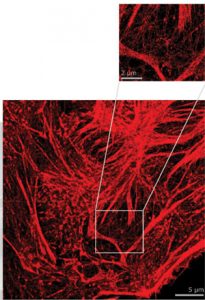
Imaged conventionally the fluorophores on the labelled structure are too close to spatially resolve. By imaging with high intensity under specific buffer conditions the fluorophores can be made to blink as they switch states. This is a rendering of all localised spots during the acquisition.
Specification Comparison
There are a handful of competitors in the single molecule/super resolution microscopy field. Contained below is a table comparing the specifications of the Nanoimager to its main competitors.
| Nanoimager | Competitor 1 | Competitor 2 | Competitor 3 | |
|---|---|---|---|---|
| Design Concept | Purpose Built | Purpose Built | Legacy Oculars | Legacy Oculars |
| Optical Path Efficiency/Aberration | Optimum/Low | Unnecessary optics/Moderate | Unnecessary optics/Moderate | Unnecessary optics/Moderate |
| Alignment | Never | Frequent | Frequent | Routinely |
| Laser Power Density (kW/cm2) | >10 | >10 | <<10 | <<10 |
| Laser Power (mW) | Up to 1000 | 1000 | 200 | 150 |
| Field of View (µm2) | 5000 Homogeneous Illumination | 400 to 1600 | 400 to 6400 | No data |
| Operation at 37°C | Yes (Entire Instrument) | Yes (Entire Instrument) | Optional (Partial Instrument) | Optional (Partial Instrument) |
| Sample Stage Reproducibility/Resolution | Piezo Inertia Drive 30nm/2nm | Stepper Motor 700nm/100nm | Piezo Based 1000nm/200nm | Piezo Ultrasonic |
| z-Travel Range (mm) | 5 | 0.1 or 0.4 | <0.5 | <0.5 |
| Software | Custom | Custom | One size fits all across microscopy range | Not single molecule localisation optimised |
| Optimised for Wide-Field smFRET | Yes | No | No | No |
| Optical Table/Infrastructure Required | No | Yes | Yes | Yes |
| Footprint/Place of Operation | <0.5m2/Any desktop | Up to 3m x 2m/special facilities | Up to 3m x 2m/special facilities | Up to 3m x 2m/special facilities |
| Ease of Use | Good | Poor | Moderate | Poor |
| 3D Super Resolution Method | Astigmatism | Bifocal | Phase ramp (2x lower resolution) | None |
| Focussing | Real time focus lock, one-shot automatic focus | Manual | Real time focus lock | Real time focus lock |
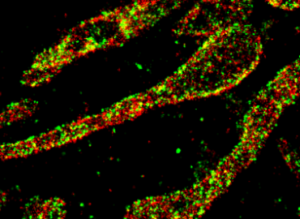
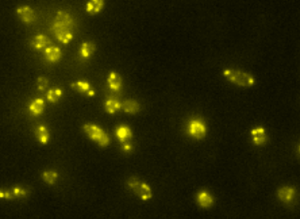
Quantitative Cellular Imaging
Molecular Mechanisms and Interactions
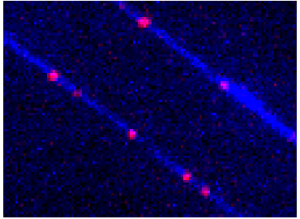
Protein Complex Assembly
Epigenetic Mapping
DNA Paint
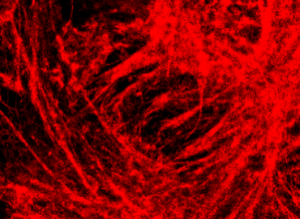
Super Resolution by Single-Molecule Localisation
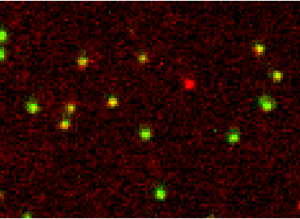
Single Molecule FRET
Exosomes and Microvesicles
Neuronal Imaging