Nanotoxicology Studies Using Tomographic Microscopy
by Desley Pitcher and Dr. Cameron Chai
Abstract
Nanotechnology has been an increasingly important topic of research since the 1980s with sub-micron research funding increasing in 2000 when US President Clinton established the National Nanotechnology Initiative to co-ordinate nanotech research.
Researchers, and now commercial organisations are producing materials such as food additives, pigments, pharmaceutics and cosmetics with nano-sized particles but much research remains to be done on the effects of these materials on human health. It is the area of nanomedicine where the technology is charging ahead as there are a variety of potential uses for nanoparticles including the exciting areas of biosensors as well as targeted and controlled drug delivery vehicles.
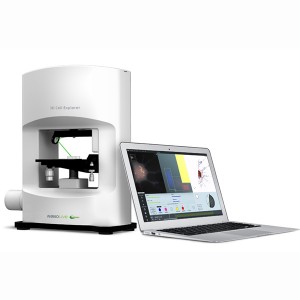
The Nanolive 3D Cell Explorer provides a unique and simple method for observing the interaction between living cells and nanoparticles.
Background
It is now well-known that materials with particle sizes in the range 1 to 100 nm have different properties to materials of the same chemistry with larger particle sizes. While the beneficial properties e.g. electronic, optical, strength etc. are being taken advantage of, the effects of these materials on human health are not well understood.
The use of these nanoparticles in the field of medicine is an exciting step forward but research is still needed to discover how these particles interact with cells and tissues and to determine whether the body will experience any toxicity. Various conformational features (shape, size, surface properties) can have an impact on a nanoparticles toxicity and it is likely that there will be variation based on cell and tissue type(1).
Finding simple and cost-effective ways to visualise the interactions of nanoparticles with cellular material is of great benefit to medical research.
3D Cell Explorer
The 3D Cell Explorer from Nanolive is a unique and novel 3D microscope that allows users to see deep into living cells without the need for labels, markers, stains, dyes or other interferences. With virtually no sample preparation required and a system that produces images in real time, you can generate high resolution images of entire cells almost instantaneously. This allows you to see and record exactly how cells behave.
The Nanolive 3D Cell Explorer is a tomographic holographic microscope which measures how light interacts with various components of the cell. These measurements are then reconstructed to produce a detailed 3D image of the cell. The images are then digitally stained using the included STEVE software package to segment out different features based on differences in refractive index. The images can also be exported to third part 3D image analysis software programs for more detailed analyses.
3D Cell Explorer for Nanotoxicity
The 3D Cell Explorer is ideally suited to nanotoxicity studies for a number of reasons. These include:
- Nanomaterials and biological materials have distinctly different refractive indices making them easy to tell apart
- Assimilation and aggregation of nanoparticles can be observed
- Cellular behaviour, including death can be monitored
- Non-invasive
- Low power illumination
- Fast acquisition
- Low photo-bleaching
- Quantitative 4D data
- Images can be captured in real time
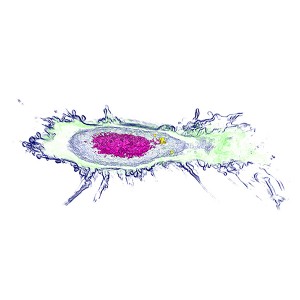
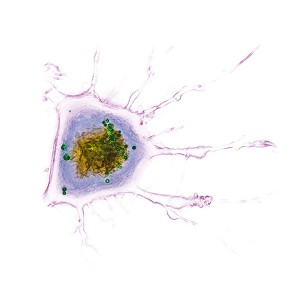
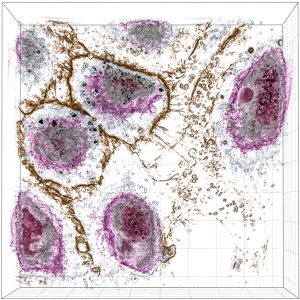
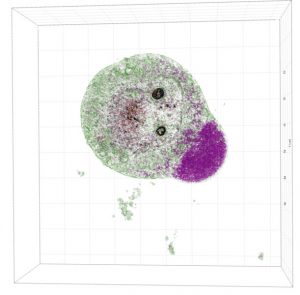
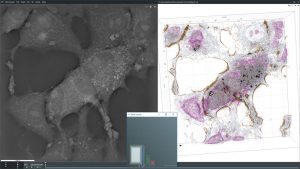
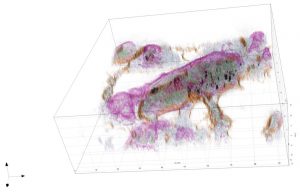
Sample Images
The images collected during this study show how these particular nanoparticles were absorbed and accumulated inside liver cells. After a period of time, the nanoparticles were found to be toxic, which resulted in the eventual apoptotic death of the liver cells.

University of Sydney researchers imaging live cells using the Nanolive 3D Cell Explorer, Dr. Wojtek Chrzanowski, Dipesh Khanal and Sally Kim (L to R).
Researchers
The images in this application note were provided by Dr. Wojtek Chrzanowski from the Faculty of Pharmacy and the Australian Institute for Nanoscale Science and Technology at the University of Sydney and is his team. They are currently conducting research on nanotoxicity, stem cell and exosome-based therapies and cell biophysics.
For more information on Dr. Chrzanowskis experiences with the Nanolive 3D Cell Explorer, visit Researchers Investigate the Toxicity of Nanoparticles Using Tomographic Microscopy.