XRD-DSC – X-Ray Diffraction with in situ Differential Scanning Calorimetry
The Rigaku XRD-DSC is able to perform in situ Differential Scanning Calorimetry measurements in an X-Ray Diffractometer. These measurements can be carried out simultaneously. This enables researchers to be able to continuously monitor changes in crystalline materials during phase changes such as polymorphic transformations, dehydration, fusion or solidification.
Key Features
- Simultaneous measurement of crystallographic and thermal changes
- Measurement temperature to 350°C (optional from -40°C)
- Measurement atmosphere air, inert gas, humidity (up to 90%RH with optional HUM-1)
Advantages
The ability to take both XRD and DSC measurements simultaneously not only saves time and labour, allowing you to streamline your workflow. Furthermore, it also provides data that can be directly correlated as it has been collected under identical experimental conditions, providing a high degree of reliability. This in turn allows thermal and crystallographic changes to be tracked simultaneously.
Applications
- Organic materials and compounds
- Medicines and pharmaceuticals
- Ceramics
- Polymers and plastics
- Electronic materials
- Battery materials
Specifications
| X-Ray Diffractometer | Ultima III, Ultima IV, SmartLAB |
| DSC Section | Heat flow type DSC ciruit and Thermo Plus 2 thermal analysis station |
| Sample Container | Made of aluminium |
| Measurement Temperature Range | Ambient to 350°C (option:low temperature measurement from -40°C) |
| Measurement Atmosphere | Air, inert gas (option: Humid atmosphere measurement from ambient to 60°C, 20 to 90% RH by connecting to the HUM-1 humidity generator) |
| 2θ Measuring Angle Range | 2θ = 5 to 40° |
| Heating and cooling rate | 0.5 to 10°C/min, temp, held constant (depending on how the measuring angle range and the fixed-rate autospeed are continued |
| Sample Size | 7.0 x 7.0 x 0.25mm |
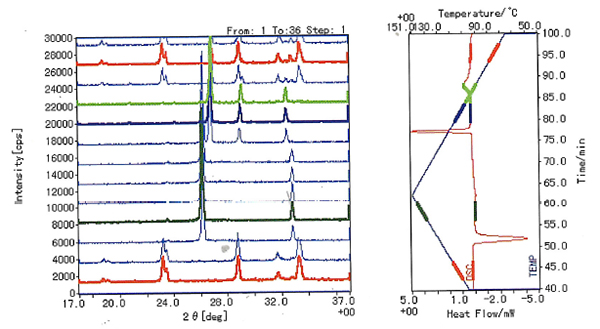
Phase Transition of Potassium Nitrate (KNO3)
Potassium Nitrate (KNO3) is a well-known ferroelectric material that is used as a thermal standard in thermal analysis.
During heating, it undergoes a phase change from orthorhombic to rhombohedral at about 128°C. During cooling it reverts back to the orthorhombic phase via an intermediate phase. This intermediate phase only appears during cooling.
- The simultaneously collected data below clearly shows the transition during heat up. This can be seen in the DSC plot where a distinct endothermic reaction takes place at about 120°C. In parallel, the XRD data clearly shows a crystallographic phase change taking place at this temperature (orthorhombic to rhombohedral)
- On cooling an exothermic reaction is observed at approximately 110°C. XRD data shows the transition from the rhombohedral phase to another (intermediate) phase.
- Upon further cooling to approximately 100°C, another smaller exothermic reaction takes place. At this point we can see the formation of the orthorhombic phase